How Groundbreaking Treatment Gives Patients Hope
- Duran Duran guitarist Andy Taylor, 62, celebrates a milestone 16th album release. The moment has added meaning because, upon his stage 4 prostate cancer diagnosis, he was facing “end-of-life” care, but a groundbreaking treatment has given him a new lease on life.
- It’s important to remember that most prostate cancer can be caught with screening exams, which include a digital rectal exam (DRE) and a protein-specific antigen (PSA) test.
- Early detection of the prostate is important as it can help reduce the risk of cancer spreading to other organs.
- Pluvicto (lutetium Lu 177 vipivotide tetraxetan) is a Food and Drug Administration (FDA)-approved cancer treatment that specifically treats patients with a type of disease called prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC).
- PSMA is a protein expressed in some prostate cancers that can be targeted with medication. Metastatic castration-resistant prostate cancer means the cancer has spread beyond the prostate and does not respond to hormone therapy.
- Plutvicto is an intravenous radioligand therapy that targets cancer cells precisely in the body. The medication combines diagnostic and therapeutic capabilities, which can identify the presence of a target or PSMA protein on a patient’s cancer cells and kill them with minimal impact on normal tissues.
Pop band Duran Duran is riding a wave of momentum following the new release of their 16th studio album since their formation in the 1970s. The milestone has added meaning for the band’s guitarist Andy Taylor, 62, who at one time did not know he would be around to celebrate this accomplishment with his bandmates after he was diagnosed with stage 4 prostate cancer. However, his groundbreaking treatment has given him a new lease on life and more opportunities to cherish life’s joyous moments.

Duran Duran’s album, “Danse Macabre,” was released on October 27 and featured a mix of covers, new songs, and classic hits. One of Taylor’s favorite hits on the track list includes “Black Moonlinght.”
Read MoreHAPPY DANSE MACABRE RELEASE DAY! It’s hard to believe the album is here, and hopefully in your hands (or streaming on your computer!)
Oh …and here’s an album unboxing video for you – no FOMO! – you can get your own now! Watch here: https://t.co/t2qATslt7S pic.twitter.com/fjFwRTb0lR— Duran Duran (@duranduran) October 27, 2023
The award-winning group was formed in 1978 and is known for songs like “Save a Prayer” and “Ordinary World.” Their journey to this milestone release involved some members’ departures and returns. Still, arguably, no journey has been more remarkable than Andy Taylor, who was once faced with end-of-life care when he was diagnosed with prostate cancer but is now thriving again thanks to the new treatment.
Taylor dedicated an Instagram post to the team behind his groundbreaking cancer treatment – Pluvicto (lutetium Lu 177 vipivotide tetraxetan) – to give him a fighting chance.
View this post on Instagram
“Having such a transformative journey, one where the generosity of spirit has carried me to a better place, enlightened me as to what my better purpose can be now that I have some life back, which is bloody amazing, by the way,” Taylor said.
RELATED: Metastatic Prostate Cancer: How Molecular Testing Can Impact Your Treatment Plan
Helping You with Prostate Cancer Resources
Andy Taylor’s Groundbreaking Treatment
Taylor had kept his cancer private until last year, just before the band was inducted into the Rock and Roll Hall of Fame.
He described his unwillingness to draw added attention to his prostate cancer as “Duran denial,” referring to the group’s tendency to “plow through with good strength of character.” However, his earlier treatments sometimes made him feel dizzy, even during performances.
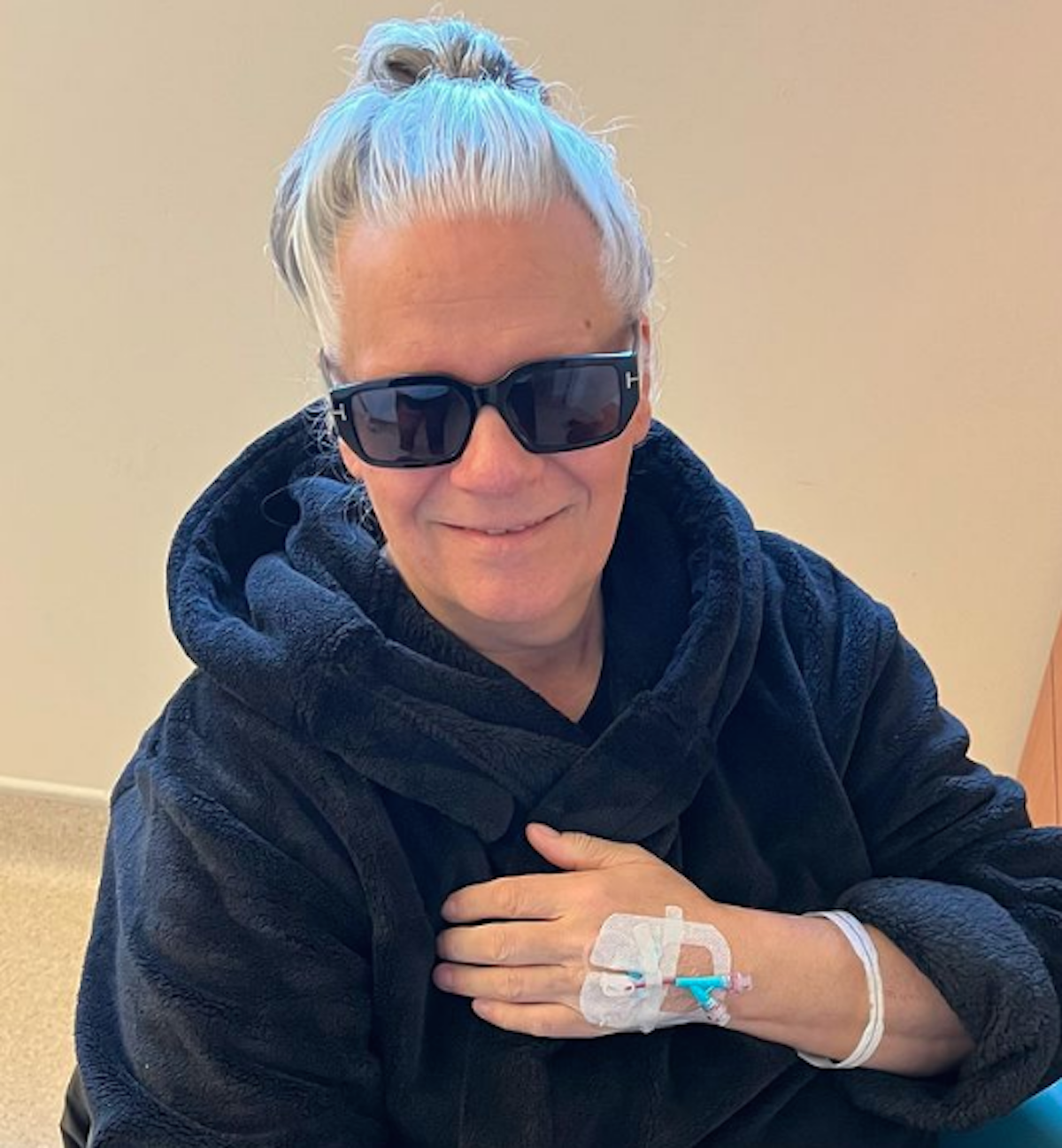
“I’d get this dizziness and have to stop and hold onto something. I’m the kind of person who won’t quit until I’m absolutely on my knees, but I realized, ‘I’m going to f—k this up for everybody else.’ So, I thought it was best to leave them to do it because they were on the road and were in good shape,” Taylor explained to Yahoo Entertainment.
Some of Taylor’s side effects stemmed from the chemo he was undertaking. At one point, when he learned his cancer had metastasized to stage 4, he was facing “palliative, end-of-life care.”
RELATED: Clearing Up Misconceptions About Palliative Care
Sir Chris Evans and his [Cancer Awareness Trust] team contacted Taylor, offering him a new treatment option. The new option was an FDA-approved radiopharmaceutical drug called Pluvicto (lutetium Lu 177 vipivotide tetraxetan). Pluvicto may be helpful for anyone with prostate cancer that has spread beyond the prostate and has not responded to hormone therapy.
“Unlike chemotherapy, the quality of life you get from this is staggering. Like, wow. It can add three to five years to your life – and I’m going for the five because they think I can make that. I am still terminal because there’s nothing curative for [stage 4 prostate cancer]. But I’ve beat the odds with this treatment,” Taylor said.
The FDA approved Plutvicto (lutetium Lu 177 vipivotide tetraxetan) in March 2022. It specifically treats patients with prostate-specific membrane antigen (PSMA) – positive metastatic castration-resistant prostate cancer (mCRPC).
PSMA is a protein that is expressed in some prostate cancers and can be specifically targeted with medication. Metastatic castration-resistant prostate cancer, more specifically, means the cancer has spread beyond the prostate and does not respond to hormone therapy, which is aimed at impeding cancer growth that relies on hormones to flourish.
Plutvicto is an intravenous radioligand therapy that targets cancer cells precisely in the body. The medication combines diagnostic and therapeutic capabilities, which can identify the presence of a target (PSMA) on a patient’s cancer cells and then treat it directly with minimal impact on normal tissues.

“The approval of lutetium is a major step in the development of personalized treatment for advanced prostate cancer,” Dr. David Penson of Vanderbilt University Medical Center told SurvivorNet.
“This agent specifically targets PSMA-positive metastasis and represents the first theranostic agent for use in castration-resistant metastatic prostate cancer,” Dr. Penson continued.
To use Plutvicto, patients must first be given a PET scan using a special imagine agent that helps better identify cancer cells within the body that are PSMA positive.
“If the patients have PSMA-positive metastatic lesions on PET, then they would be eligible for the therapy. This combination of a (therapy that can deliver radiation to target cells) with an imaging biomarker positivity is a great example of precision medicine or what we call in nuclear medicine, theranostics (therapeutics + diagnostics),” Dr. Ghassan El-Haddad, associate member of the Diagnostic Imaging and Interventional Radiology Department of Moffitt Cancer Center explains.
Like most treatments, Plutvicto has side effects. The most common adverse reactions may include:
- Fatigue
- Dry mouth
- Nausea
- Anemia
- Decreased appetite
- Constipation
- Screening for Prostate Cancer
(What to Know About Prostate Cancer Screening)
Most prostate cancer is caught with screening examinations, which include a digital rectal exam (DRE) to check for lumps. The protein-specific antigen (PSA) test is another effective means of screening. This is a simple blood test that screens for prostate cancer. It looks for larger amounts of protein-specific antigen in the blood. An elevated PSA test does not always mean you have prostate cancer. It could also indicate that your prostate is enlarged, which is common, or could signal an infection or inflammation.
Prostate cancer does not always behave the same in every man it impacts. The cancer can be considered “low-risk” and can be slow-growing, and treatment might not be necessary. In other men, the cancer may grow faster or be more aggressive, requiring more immediate treatment. Because of this, there is some debate about screening.
WATCH: When to screen for prostate cancer.
The United States Preventive Services Taskforce recommends that men at average risk between the ages of 55-69 should talk with their doctor about the pros and cons of prostate cancer screening. Most doctors agree that men over 70 do not need screening.
SurvivorNet experts suggested that men consider factors like their family history, genes, and age when deciding whether and when to screen.
Other warning signs of prostate cancer are:
- Blood in your urine
- Trouble getting an erection
- Pain or burning when you urinate
- Pain in your back, hips, thighs, or other bones
- Unexplained weight loss
- Fatigue
Questions to Ask Your Doctor
Here are some questions you may consider asking your doctor about your risk of developing prostate cancer:
- How does my family history affect my risk of developing prostate cancer?
- Are there tests available to determine my genetic risk of developing prostate cancer?
- Based on my history, genetic test results, and other factors, when do you recommend I begin screening for prostate cancer?
- How can I prepare for prostate cancer screening?
Learn more about SurvivorNet's rigorous medical review process.

